Structure of the signal recognition particle by electron microscopy
Andrews, D.W., Walter, P. and Ottensmeyer, F.P., Structure of the signal recognition particle by electron microscopy. (1985) Proc Natl Acad Sci., 82:785-790.
Read MoreElectron microscopic visualization of the side chains of the poly-L-lysine alpha helix.
Harauz, G., Andrews, D.W. and Ottensmeyer, F.P., Electron microscopic visualization of the side chains of the poly-L-lysine alpha helix. (1983) Ultramicroscopy, 12:59-64.
Read MoreElectron microscopy of the Poly-L-lysine alpha helix
Andrews, D.W. and Ottensmeyer, F.P., Electron microscopy of the Poly-L-lysine alpha helix. (1982) Ultramicroscopy, 9:337-348.
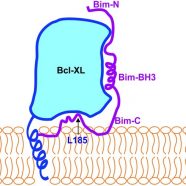
Read MoreBim escapes displacement by BH3-mimetic anti-cancer drugs by double-bolt locking both Bcl-XL and Bcl-2
Liu Q, Oesterlund EJ, Chi X, Pogmore J, Leber B, Andrews D.W., Bim escapes displacement by BH3-mimetic anti-cancer drugs by double-bolt locking both Bcl-XL and Bcl-2. (2019). Elife. 8:e37689. doi: 10.7554/eLife.37689.
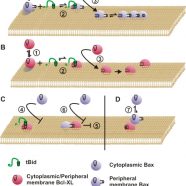
Read MoreBcl-XL inhibits membrane permeabilization by competing with Bax
Billen L.P., Kokoski C.L., Lovell J.F., Leber, B., and Andrews, D.W., Bcl-XL inhibits membrane permeabilization by competing with Bax. (2008) PLoS Biol 6(6): e147. [Featured in a mini-review in the same issue and in Research Highlights, Nature Immunology, August 2008, 9:837; Faculty 1000 Biology: http://www.f1000biology.com/article/id/1123833/evaluation].
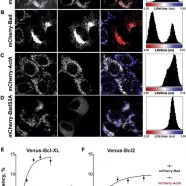
Read MoreDifferences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells
Aranovich, A., Liu, Q., Collins, T.J., Geng F., Dixit S., Leber B., and Andrews, D.W., Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. (2012) Mol Cell., 45:754-63.
Read More
 Find David Andrews Lab Plasmids
Find David Andrews Lab Plasmids